
Thermal Science & Engineering
Basic Concepts in Thermal Engineering
Study of Thermodynamics
Thermodynamics is a science that involves Heat, Work and Power and the related changes in the working substance properties.
System – A system is a collection of matter within prescribed and identifiable boundaries.
In other words a system is a quantity of matter or a region in space ,where we choose for study .
The mass or region outside the system is called surroundings.
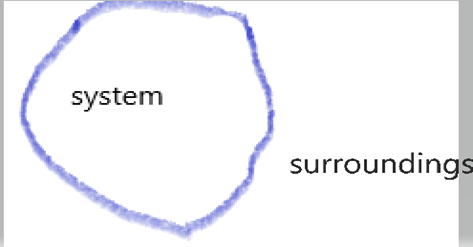
System Boundary
The Boundary can be physical or imaginary,fixed or movable.
Systems are classified basically in to open, closed, or isolated based on whether constant volume or mass in space is chosen for study.
Open System (or Control Volume )
System with fixed volume in space both mass and energy transfer occur across the boundary.
Eg : Nozzle, Turbine, Compressor, Pipe.
Closed System (or Control Mass )
In closed systems, only energy can be exchanged, but not matter. So mass of closed system remains constant. The energy transfer can be in the form of heat and work
Eg : Reactions in a closed vessel permits exchange of heat across the boundary.
Isolated System
It is same as the closed system, But doesn’t allow the energy transfer.
State Functions, Path Functions, Thermodynamic Process
Any Characteristics of a system is called property.
Eg: Pressure, Temperature, Volume, Mass, Velocity
Properties can be classified as extensive or intensive.
Intensive
Properties Independent of the mass of the system is called Intensive
Eg: Pressure, Temperature, Density
Extensive
Properties dependent on the size or Extent of the system is called Extensive.
Eg : Total mass, Total Volume, Specific volume.
State Functions
State Function Describe an aspect of a chemical system.
Pressure, Temperature, Volume, No of Moles or Atom, Internal Energy ( E),Enthalpy( H),Entropy (S) and Gibbs Free Energy (G) are State Functions.It depends on the initial and Final State of the system, Not depending on the path or the Path.
Path Functions
Path functions are those quantities that depend on the path taken to achieve the final state of the system.
Eg : Heat or Work.
Work is the Suitable Example —Work done in climbing a hill by bus and Work done in climbing the same hill by walking is different.
Thermodynamic Process.
A thermodynamic Process is a path in which the state of the system changes and their property varies.
Six main types of Thermodynamic Processes are,
Adiabatic – No heat transfer occurs one fluid to another.
Isothermal – Temperature of the system remains constant.
Isentropic – The entropy of the system remains constant.
Isobaric – The pressure of the system remains constant.
Isochoric – The volume of the system remains constant.
Isenthalpic – Enthalpy of the system remains constant.
Energy and Energy Transfer
Energy can exit in any of the forms Such as Thermal, Mechanical, Electrical, Chemical and Nuclear.
Even mass can be considered a form of Energy, Energy is interconvertible. In closed system (where mass cannot transfer only energy transfer occurs. It happens either in the form of heat and work.
Control Volume – Energy transfer is either by a heat, work and mass flow.
Macroscopic and Microscopic forms of energy
Macroscopic – Internal energy of the system related to molecular structure.
Macroscopic – Influenced by external effects .(Eg : Kinetic energy, Potential energy etc. all acquired by the external effects of the system).
Total Energy of a system , E = U+ K.E+ P.E
U – Internal Energy
K.E – Kinetic Energy
P.E – Potential Energy
Enthalpy – The amount of Energy absorbed or released during a reaction. During a reaction some bonds are broken and some new bonds are formed.
Change in Enthalpy during a chemical reaction is
H = H (Products) – H(Reactants)
Exothermic reaction – Heat is released during the reaction, H is – ve.
Endothermic reaction – Heat is absorbed during the reaction, . H is +ve.
Enthalpy , H = E + PV
E – Internal energy
PV – Work
In closed system ( control mass ) , and a non flow process,
Q = m Cv dt
Cv – Sp heat at constant volume.
Specic Heat – The sp. Heat is defined as the energy required to raise the temp. of unit mass of substance by 1 degree.
Specific Heat at constant Pressure – Cp
Specific heat at constant Volume – Cv
Cp > Cv.
Q = m Cv T. ( Constant volume heat addition )
Q = m Cp T ( Constant pressure heat addition )

